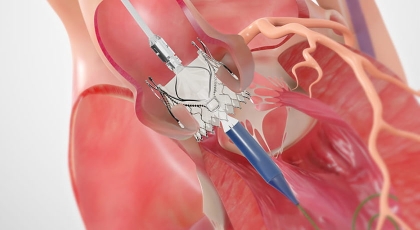
Resources*
Find the latest articles, resources, and presentations
about aortic regurgitation here.
*Indications: The JenaValve Trilogy Heart Valve System is indicated for use in patients with native symptomatic, severe aortic regurgitation (AR) or symptomatic, severe aortic stenosis (AS), who are judged by a Heart Team (including a cardiac surgeon), to be at high or greater risk for surgical aortic valve replacement (AVR), with an STS score ≥ 8% at 30 days, or other comorbidities (e.g., porcelain aorta, frailty, chest wall irradiation) that are not captured by the STS risk calculator.
Contraindications: The JenaValve Trilogy Heart Valve System is contraindicated for use in patients who have known hypersensitivity or contraindication to Nitinol (titanium and/or nickel), an anti-coagulation/anti-platelet regimen or contrast medium that cannot be managed with premedication, or who have active bacterial endocarditis or other active infections.
US: CAUTION – Investigational Device. Limited by Federal (or United States) law to investigational use.